Knee replacement lawsuits focus on device complications experienced by patients. Many of these complications are caused by defective design or manufacturing. Patients who suffer severe medical device complications can seek legal help. Injured patients can win money to pay for device revision or replacement, physical therapy and related treatment.
Why People Are Filing Knee Replacement Lawsuits
Knee replacement lawsuits occur when knee implants fail or are defective. Patients may receive knee implants during knee replacement surgery, also known as total knee arthroplasty. If the implants are defective, patients may experience negative side effects or implant failure.
Depending on the severity of side effects, recipients of faulty knee implants may require costly medical care or additional surgeries. Knee implant lawsuits allow such patients to hold the manufacturer financially responsible for defective knee implants.
Why People Are Filing Exactech Knee Replacement Lawsuits
Exactech knee replacement lawsuits stem from an ongoing recall caused by manufacturing mistakes. These manufacturing mistakes may make some knee replacements fail early. A number of patients who received these implants are having issues with them. Some of these difficulties are forcing patients to have corrective surgery, which is also called revision surgery.
Patients affected by faulty Exactech implants may be able to sue for legal damages. Compensation for legal damages might help patients pay for treatment related to their recalled knee implants.
Why Are Some Exactech Knee Replacement Components Recalled?
Exactech knee implants have been recalled due to faulty packaging that was used during shipment and storage. Company officials say implants lacked an important packaging barrier. This barrier was supposed to keep oxygen from coming in contact with the inserts. However, it lacked a special layer with additional protection against oxygen.
Without the extra protective layer, oxygen could pass through the implant packaging. The oxygen could then cause certain components to break down earlier than expected. Patients receiving these implants could experience complications and need to have revision surgery.
Exactech recently issued a recall notice for Optetrak, Optetrak Logic and Truliant knee replacements. According to this notice, doctors have implanted more than 140,000 of these knee devices since 2004. This means tens of thousands of patients may have problems related to Exactech knee implants.
Understanding Knee Replacement Lawsuits
Between 2012 and 2017, more than 744,000 knee placement and revision surgeries took place in the United States. Knee replacement surgeries are generally considered safe. However, painful and debilitating complications can arise. Many of these problems occur because of defective knee devices. Some devices have been recalled by manufacturers because they caused so many problems for patients.
Medical device implants are difficult to repair, replace or remove. Patients who suffer serious side effects may require additional surgeries or other treatments.
Knee replacement lawsuits claim that medical device companies failed to warn patients about potential dangers. Plaintiffs seek legal damages to pay for treatment, costs of the device, lost income, and other expenses related to problems with the device.
Complications Mentioned in Knee Device Lawsuits
- Pain and aching
- Inflammation
- Infection
- Swelling
- Loosening of the joint
- Clicking or popping noises
- Mobility problems
- Metal allergy/sensitivity
- Metallosis
Knee Replacement Recalls
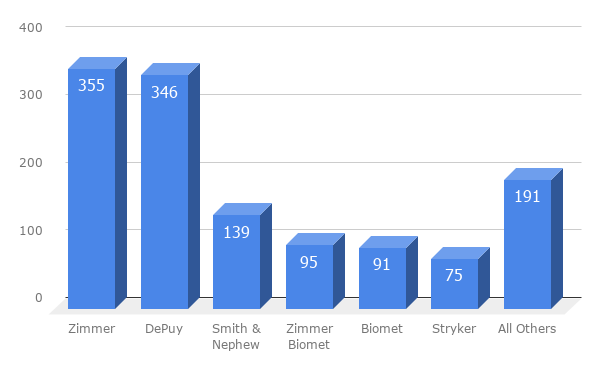
At the heart of many artificial knee lawsuits are design or manufacturing problems. The U.S. Food and Drug Administration keeps a recall database for medical devices. According to the agency's database, there were nearly 1,300 recalls on knee replacement systems or components between 2003 and 2019.
The companies with the most recalls have also faced significant numbers of lawsuits:
- Zimmer - 355 recalls
- DePuy - 346 recalls
- Smith & Nephew - 139 recalls
- Biomet - 91 recalls
- Exactech - 47 recalls
Exactech has issued at least 47 recalls since 2003. The most recent Exactech recall applies to more than 140,000 implanted knee and ankle replacements.
Knee Implant Recalls From 2003 to 2019
Source: U.S. Food and Drug Administration Recall Database
Current Knee Replacement Litigation
Over the years, many lawsuits have been filed against knee device makers including:
- Exactech
- Stryker, Smith & Nephew
- Wright Medical
- Biomet
- Zimmer
Due to the volume of lawsuits, many of these cases were consolidated into multidistrict litigation. Plaintiffs seek legal damages related to medical bills, pain and suffering related to their faulty knee implants.
Multidistrict litigation (MDL) is another method that courts use to merge lawsuits. Unlike a class action lawsuit, individual cases remain separate in an MDL. However, certain phases of litigation are combined for all the lawsuits. This speeds up the process of trying hundreds of similar cases.
Exactech Knee Lawsuits
Exactech knee replacements have been involved in litigation for years now. In fact, the 2021 Exactech recall covers Optetrak devices, which also garnered lawsuits as far back as 2017. The implant failures named in those lawsuits may have originated from the same packaging issue driving the current recall.
The 2022 Exactech knee recall covers Optetrak, Optetrak Logic and Truliant knee replacements. A missing oxygen barrier in the device packaging may have caused these implants to break down. Early deterioration of Exactech knee implants may cause:
- Joint grinding, instability or swelling
- Loss of weight-bearing ability
- Prolonged pain
These problems may require patients to undergo revision surgery, which can be costly. As a result, patients harmed by faulty Exactech knee implants are suing the manufacturer. Lawsuit verdicts or settlements may help these patients cover revision surgery and other related costs.
Knee replacement lawyers estimate Exactech knee lawsuits may yield settlements or verdicts ranging from $70,000 to $400,000. Anyone considering an Exactech lawsuit should speak with an attorney experienced in faulty medical implants. These lawyers can help potential plaintiffs determine the best legal approach for their case.
Zimmer Knee Lawsuits (MDL 2272)
In 2010, Zimmer issued recalls for multiple knee components in its NexGen Knee Replacement system. As details about the NexGen implant problems were made public, people who suffered pain, discomfort and immune system reactions to their knee implants began filing lawsuits against the company.
In 2011, federal lawsuits against Zimmer NexGen knee devices were consolidated into MDL 2272. More than 1,700 Zimmer lawsuits were filed in the MDL before trials began in 2015.
Several of the first few cases selected for bellwether trials were dismissed before they went before a jury. By June 2016, a single bellwether trial was complete and 15 others had been dismissed.
In June 2016, Judge Rebecca R. Pallmeyer issued a case management order that threw out cases regarding claims other than device defectiveness.
This "Lone Pine" order required claimants to "provide evidence or certification of loosening of a NexGen Flex femoral component, a 5950 NexGen MIS Stemmed tibial component, or any other tibial component implanted with a NexGen Flex femoral component." Claimants who failed to do so within the specified time frame had their cases dismissed.
MDL 2272 was closed in October 2019.
Zimmer Lawsuit Settlements
In January 2018, Judge Pallmeyer ordered both sides of the NexGen Knee Systems lawsuits to attend arbitration. Arbitration is often used in an attempt to reach a settlement. In February, the two sides came back with a proposed settlement offer. All pending cases were put on hold.
In June 2018, the plaintiffs' lawyers announced a settlement agreement with Zimmer Biomet over NexGen lawsuits.
According to Zimmer Biomet's 2018 Annual Report, the company informed the court it was finalizing a settlement that would resolve all but six of the outstanding cases in the MDL. Details of the settlement remain confidential.
Other Knee Replacement Lawsuits
Long before the NexGen lawsuits, Sulzer Medica paid $1 billion to settle about 4,000 knee device lawsuits. This settlement came before the company was acquired by Zimmer in 2003. Problems with the production of Sulzer's Natural Knee II Tibial Baseplate caused a small amount of oil to be left on the device. This led to post-implantation problems and adverse effects following the surgery. The company had issued a recall on the knee component in 2000.
Zimmer Biomet currently faces the most lawsuits, but other companies have also faced individual and class action lawsuits due to defective products. Some of the brands involved in complex litigation include:
- DePuy Synthes Attune Knee System (Johnson & Johnson)
- Exactech Optetrak Knee Replacement
- Duracon Unicompartmental Knee
- Stryker ShapeMatch Cutting Guides (not a knee joint component itself, but a tool used in knee surgery)
Individuals who have suffered complications from these devices should consider legal action.
Product liability lawsuits related to knee replacements differ from medical malpractice lawsuits.
Are Knee Replacement Lawsuits Still Being Filed?
Patients are currently filing knee replacement lawsuits related to the Exactech knee replacement recall. And, defective medical devices still face regular recalls. It is possible more recall lawsuits could be filed in the future.
If you received a faulty Exactech knee replacement, you should speak with a doctor and a lawyer right away. A doctor can help you understand your medical options. A knee replacement lawyer can help determine the best approach to legal recourse. A reputable lawyer can also take your case on a contingency basis, meaning you will have zero upfront costs. In this situation, you will only pay legal fees if your case reaches a settlement or verdict.